Summary of current evidence as found on the 4 Resources Pages
For new literature reviewed for the 2020 RBR, the level of evidence has been evaluated where possible using both the former Canadian Task Force on Preventive Health Care and the GRADE classification systems.”
Strength of recommendation retains the previous scheme using “Good, Fair, and Inconclusive evidence/Consensus."
The button indicates links to Parent Resources on this topic.
GROWTH
- Important: Correcteds age should be used at least until 24 to 36 months of age for premature infants born at < 37 weeks gestation.
- Measuring growth: The growth of all term infants, both breastfed and non-breastfed, and preschoolers should be evaluated using Canadian growth charts from the 2006 World Health Organization Child Growth Standards (birth to 5 years) with measurement of recumbent length (birth to 2–3 years) or standing height (≥ 2 years), weight, head circumference (birth to 2 years) and calculation of BMI (2–5 years). WHO Growth Charts Adapted for Canada (DC) Growth Monitoring (CTFPHC) Optimal growth monitoring (CPS)
NUTRITION
NUTRITION: Nutrition for healthy term infants (NHTI): 0–6 months | 6–24 months | NutriSTEP® | Overview NHTI 0–6 months (CPS) | 2019 Nutrition Guidelines (ODPH) | Dietitians of Canada
- [ ** ](3)
Breastfeeding: Exclusive breastfeeding is recommended for the first six months of life for healthy term infants. Introduction of solids should be led by the infant's signs of readiness-a few weeks before to just after 6 months. Breast milk is the optimal food for infants, and breastfeeding (with complementary foods) may continue for up to two years and beyond unless contraindicated. Breastfeeding may reduce gastrointestinal and respiratory infections and helps to protect against SIDS. Maternal support (both antepartum and postpartum) increases breastfeeding and prolongs its duration. Early and frequent mother- infant contact, rooming in, and banning handouts of free infant formula increase breastfeeding rates.
- Breastmilk storage: 2019 Nutrition Guidelines (ODPH) page 8
- Ankyloglossia and breastfeeding (CPS)
- Maternal medications when breastfeeding: Drugs and Lactation Database (TOXNET)
- Weaning: Weaning from the breast (CPS)
- [ ** ](4)
Vitamin D supplementation of 400 IU/day (800 IU/day in high-risk infants) is recommended for infants/children for as long as they are breastfed. Breastfeeding mothers should consume a standard multivitamin/mineral supplement that contains vitamin D (400 IU/day).
Vitamin D supplementation (CPS) - [ ** ](5)
Infant formula: Formulas generally contain iron: 0.4mg–1.3mg/100ml. Discourage the use of homemade infant formulas.
- Formula composition and use Alberta Health Services Compendium and Summary Sheet
- Infant Formula: What you need to know (Best Start) | Preparation Video and Tip sheets (Best Start)
Milk consumption range is consensus only & is provided as an approximate guide.
Soy-based formula is not recommended for use in cow milk protein allergy or in preterm infants, and may interfere with absorption of T4 replacement therapy in infants with congenital hypothyroidism. Soy-based formulas (AAP)
- (6)
Milk consumption range is consensus only & is provided as an approximate guide.
Dietary fat content: Restriction of dietary fat during the first 2 years is not recommended since it may compromise the intake of energy and essential fatty acids, required for growth and development. After 2 years, a gradual transition begins from a high fat milk diet to a lower fat milk diet, as per Canada’s Food Guide.
- (7)
Soy-based formula is not recommended for routine use in term infants as an equivalent alternative to cow’s milk formula, or for cow milk protein allergy, and is contraindicated for preterm infants. CPS Position Statement
- [ ** ](89)
Avoid all sweetened fruit drinks, sport-drinks, energy drinks and soft-drinks; restrict fruit juice consumption to a maximum of 1/2 cup (125 mL) per day. Limit the consumption of prepared food and beverage products that are high in sugar content.
Limit/avoid consuming highly processed foods that are high in dietary sodium. Dietary sodium (CPS)
- [ ** ](8)
- [ ** ](9)
Introduction to solids: A few weeks before to just after 6 months, guided by infant’s readiness (CPS Caring for Kids), start iron containing foods to avoid iron deficiency. A variety of soft texture foods, ranging from purees to finger foods, can be introduced.
Allergenic foods: For all infants, including those at high risk for allergies, allergenic foods (especially eggs and peanut products) can be introduced with other solids around 6 months, but not before 4 months, as guided by the infant’s signs of readiness. Once allergenic solids are introduced, they should be fed a few times a week to maintain tolerance.
Timing of introduction (CPS) | Allergy check | Food Allergy Canada - [ ** ](10)
Iron containing foods: At ~6 months, start iron containing foods to avoid iron deficiency.
(11)Allergenic foods: Delaying the introduction of priority food allergens is not currently recommended to prevent food allergies, including for infants at risk of atopy. Dietary exposures & allergy prevention (CPS)
- [ ** ](12)
Avoid honey until 1 year of age to prevent botulism.
- [ ** ](14)
Dietary fat content: Restriction of dietary fat during the first 2 years is not recommended since it may compromise the intake of energy and essential fatty acids, required for growth and development. After 2 years, a gradual transition begins from a high fat milk diet to a lower fat milk diet, as per Canada's Food Guide.
Promote family meals with independent/self-feeding while offering a variety of healthy foods. NHTI: 6-24 months
- [ ** ](15)
Promote family meals with independent/self-feeding while offering a variety of healthy foods. NHTI: 6–24 months
- [ ** ](16)
Vegetarian diets: Vegetarian diets in children and adolescents (CPS)
- [ ** ](17)
Fish consumption: 2 servings/week of low mercury fish: Fish consumption and mercury (HC)
ENVIRONMENTAL HEALTH
Global Climate Change and Health (CPS) | Health and Environment: (CPCHE) | (AAP)
- (37)
Second-hand smoke/E-cigs/Cannabis exposure: There is no safe level of exposure. Advise caregivers to stop smoking and/or reduce second-hand smoke exposure, which contributes to childhood respiratory illnesses, SIDS and neuro-behavioural disorders. Offer smoking cessation resources. Educate parents on the health risks and harms associated with e-cigs and cannabis (including edibles), and on safe storage. Cannabis (CPS)
- [ ** ](38)
Sun exposure/sunscreens: Minimize sun exposure. Wear protective clothing, hats, properly applied sunscreen with SPF ≥ 30 for those > 6 months of age.
Insect bites/repellents: Prevent insect bites. No DEET in < 6 months; 6–24 months 10% DEET apply max once daily; 2–12 years 10% DEET apply max TID. Preventing mosquito and tick bites (CPS)
- [ ** ](39)
Pesticides: Ask about pesticide use and storage at home; avoid exposure. Wash all fruits and vegetables that cannot be peeled. Food additives and child health (AAP) | Pesticide Exposure in Children (AAP)
- [ ** ](40)
Lead: There is no safe level of lead exposure in children. Evidence suggests that low blood lead levels can have adverse health effects on a child’s cognitive function.
Blood Lead Screening is recommended for children who:
- in the last 6 months lived in a house or apartment built before 1960;
- live in a home with recent or ongoing renovations or peeling or chipped paint;
- have a sibling, housemate, or playmate with a prior history of lead poisoning;
- live near point sources of lead contamination;
- have household members with lead-related occupations or hobbies;
- are refugees aged 6 months–6 years, within 3 months of arrival and again in 3–6 months.
- have emigrated or been internationally adopted from a country where population lead levels are higher than in Canada.
- are at risk of lead exposure from water pipes.
- require diagnostic investigations for neurodevelopmental delays/disorders
Websites about environmental issues:
INJURY PREVENTION
INJURY PREVENTION: In Canada, unintentional injuries are the leading cause of death in children and youth. Most of these preventable injuries are caused by motor vehicle collisions, suffocation, drowning, fire, poisoning, and falls. Injury deaths in Canada (PHAC). Unexplained injuries (e.g. fractures, bruising, burns) or injuries that do not fit the rationale provided or developmental stage raise concern for child maltreatment.
- [ ** ](20)
Transportation in motorized vehicles including cars, ATVs, snowmobiles, etc.:
Transport Canada | Child passenger safety (AAP) | Preventing ATV injuries (CPS) | Snowmobile safety (Caring for Kids CPS)- Never leave a child unattended in a vehicle. Those < 13 years should sit in the rear seat, away from all airbags.
- Car seats: Install and follow size recommendations as per specific car seat model, and keep in each stage as long as possible, until the weight and height limit of the seat is reached: Infant/toddlers in a rear-facing car seat; Children who weigh at least 10 kg in a forward-facing seat with a harness; Children who weigh at least 18 kg in a booster seat. Then use properly fitted lap and shoulder belt in the rear seat for children taller than 145 cm (4' 9") and < 13 years. Replace car seat if in a collision.
- Children and youth younger than 16 years of age should not operate an ATV or a snowmobile, including youth models.
- [ ** ](21)
Bicycle: wear bike helmets and advocate for helmet legislation for all ages. Replace if heavy impact or damage. Bicycle helmet legislation (CPS)
- [ ** ](27)
Safe sleeping environment: Joint statement (CPS/CFSIDS/CICH/HC/PHAC) | 2016 task force on SIDS (AAP)
- Sleep position, bed sharing, and SIDS: Healthy infants should be positioned on their backs on a firm surface for every sleep. Counsel parents on the dangers of other contributory causes of SIDS such as bed sharing, overheating, maternal smoking, 2nd hand smoke, alcohol, or illicit or sedating drug use
- Positional plagiocephaly: While supine for sleep, the orientation of the infant’s head should be varied to prevent positional plagiocephaly. Sleep positioners should not be used. After umbilical cord stump has detached, infants should have supervised tummy time while awake. Positional plagiocephaly (PCH)
- Crib safety/Room sharing: Infants should sleep in a crib, cradle, or bassinette that meets Health Canada regulations, is located in parents’ room for the first 6 months of life, and is without soft objects, loose bedding, or similar items inside.
- Swaddling: Proper swaddling of the infant may promote longer sleep periods but could be associated with adverse events (hyperthermia, SIDS, or development of hip dysplasia) if misapplied. A swaddled infant must always be placed supine with free movement of hips and legs, and the head uncovered. Swaddling is contraindicated once baby shows signs of attempting to roll. Risks and Benefits of Swaddling (AJMCN)
- [ ** ](28)
Pacifier use: Counsel on safe and appropriate use. Pacifiers may decrease risk of SIDS and should not be discouraged in the 1st year of life after breastfeeding is well established, but should be restricted in children with chronic/recurrent otitis media. Recommendations for pacifier use (CPS)
- [ ** ](23)
Choking: Avoid hard, small and round, smooth, and sticky solid foods until age 4 years. Encourage child to remain seated while eating and drinking. Use safe toys, follow minimum age recommendations, and remove loose parts and broken toys. Preventing choking and suffocation in children (CPS)
- [ ** ](22)
Drowning: Prevention of drowning (AAP)
- Bath safety: Never leave a young child alone in the bath. Do not use infant bath rings or bath seats.
- Water safety: Recommend adult supervision, training for adults, 4-sided pool fencing with self-closing and- latching gates, lifejackets, swimming lessons, and boating safety to decrease the risk of drowning
- [ ** ](24)
Burns: Install smoke detectors in the home on every level. Keep hot water at a temperature < 49oC. Be vigilant with hot liquids on counter-tops.
- [ ** ](25)
Poisons and other toxins: Keep medicines and cleaners, and other toxic substances locked up and out of child’s reach. Have Poison Control Centre number (PCC#) handy. Use of ipecac is contraindicated in children. Install carbon monoxide detectors.
- [ ** ](26)
Falls: Assess home for hazards – never leave baby alone on change table or other high surface; use window guards and stair gates. Baby walkers are banned in Canada and should never be used. Ensure stability of furniture and TV. Advise against trampoline use at home. Trampoline safety (AAP)
- [ ** ](29) Firearm safety: Advise on removal of firearms from home or safe storage to decrease risk of unintentional firearm injury, suicide, or homicide. Prevention of firearm injuries (CPS)
OTHER
- [ ** ](44)
Advise parents against using OTC cough/cold medications: Treating cough and cold (CPS)
- [ ** ](45)
Complementary and alternative medicine (CAM): Questions should be routinely asked about the use of complementary and alternative medicine, therapy, or products, especially for children with chronic conditions. Natural Health Products (Caring for kids, CPS); Homeopathy (CPS); Chiropractic care (PCH)
- [ ** ](46)
Fever advice/thermometers: Fever ≥ 38oC in an infant < 3 months needs urgent evaluation. Ibuprofen and acetaminophen are both effective antipyretics. Acetaminophen remains the first choice for antipyresis under 6 months of age; thereafter ibuprofen or acetaminophen may be used. Alternating acetaminophen with ibuprofen for fever control is not recommended in primary care settings as this may encourage fever phobia, and the potential risks of medication error outweigh measurable clinical benefit. Fever in the returning child traveller (CPS) | Fever and temperature taking (Caring for Kids CPS)
- [ ** ](47)
Footwear: Shoes are for protection, not correction. Walking barefoot develops good toe gripping and muscular strength. Footwear for children (CPS)
- [ ** ](48)
Oral Health - Smiles for Life
- Teething: Discomfort can be managed by providing gum massage with a cold facecloth/teething ring and appropriate use of oral analgesics. E.g. acetaminophen (all ages), or ibuprofen if ≥ 6 mos. Anaesthetics/numbing gels and teething necklaces are contraindicated. Dental devel (CDA) | Homeopathic teething products (FDA)
- Dental Cleaning: As excessive swallowing of toothpaste by young children may result in dental fluorosis, children under 3 years of age should have their teeth and gums brushed twice daily by an adult using either water (if low risk for tooth decay) or a rice grain sized portion of fluoridated toothpaste (if at caries risk). Children 3–6 years of age should be assisted during brushing and only use a small amount (e.g. pea-sized portion) of fluoridated toothpaste twice daily. Caregiver should brush child’s teeth until they develop the manual dexterity to do this alone, and should continue to intermittently supervise brushing after children assume independence. Begin flossing daily when teeth touch.
- Caries risk factors include: child has caries or enamel defects, hygiene or diet is concerning, parent has caries, premature or LBW infant, or no water fluoridation. Canadian Risk Assessment Tool | Caries-risk assessment (AAPDA)
- To prevent early childhood caries: avoid juices/sweetened liquids and constant sipping of milk or natural juices in both bottle and cup.
- Fluoride varnish should be used for those at caries risk. Consider dietary fluoride supplements only for high risk children who do not have access to systemic community water fluoridation. Fluoride & your child (CDA)
- Consider the first dentist visit by 6 months after eruption of 1st tooth or at age 1 year.
BEHAVIOUR
Disruptive behaviour (CPS/CACAP)
Refer parents of children at risk of, or showing signs of, behavioural or conduct problems to structured parenting programs which have been shown to increase positive parenting, improve child compliance, and reduce general behaviour problems. Access community resources to determine the most appropriate and available research-structured programs. Parenting skills (EECD)
e.g., The Incredible Years®, Triple P®, Strongest Families
- [ ** ](31)
Crying: Excessive crying may be caused by behavioural or physical factors or be the upper limit of the normal spectrum. Caregiver frustration with infant crying can lead to child maltreatment/inflicted injury (head injury, fractures, bruising). The Period of Purple Crying.
- [ ** ](33)
Assess healthy sleep habits: Normal sleep (quality and quantity for age) is associated with typical development and leads to better health outcomes. Sleeping Behaviour (EECD). Recommended sleep duration per 24 hrs: 12-14 hrs (infants 4–12 months); 11-14 hrs (1–2 yrs); 10-13 hrs. (3–5 yrs); 9-12 hrs (6–12 yrs); 8-10 hrs (13–18 yrs). Turn off computer/TV screens 60 minutes before bedtime. No computer/TV screens in bedroom. Recommended amount of sleep (AASM)
- [ ** ](33)
Assess healthy sleep habits: Normal sleep (quality and quantity for age) is associated with typical development and leads to better health outcomes. Sleeping Behaviour (EECD). Recommended sleep duration per 24 hrs: 12-14 hrs (infants 4–12 months); 11-14 hrs (1–2 yrs); 10-13 hrs. (3–5 yrs); 9-12 hrs (6–12 yrs); 8-10 hrs (13–18 yrs). Turn off computer/TV screens 60 minutes before bedtime. No computer/TV screens in bedroom. Recommended amount of sleep (AASM)
PARENTING/DISCIPLINE
Supporting Positive parenting (CPS)
Inform parents that warm, responsive, flexible, and consistent discipline techniques are associated with positive child outcomes. Over reactive, inconsistent, cold, and coercive techniques are associated with negative child outcomes. Use of any physical punishment including spanking should be discouraged in all ages. Effective discipline for children (PCH)
Refer parents of children at risk of, or showing signs of, behavioural or conduct problems to structured parenting programs which have been shown to increase positive parenting, improve child compliance, and reduce general behaviour problems. Access community resources to determine the most appropriate and available research-structured programs. Parenting skills (EECD)
e.g., The Incredible Years®, Triple P®, Strongest Families
HIGH RISK INFANTS/CHILDREN/PARENTS/CAREGIVERS/FAMILIES
- [ ** ](84)
Maternal depression: Physicians should have a high awareness of maternal depression, which is a risk factor for the socio-emotional and cognitive development of children. Although less studied, paternal factors may compound the maternal-infant issues. Maternal depression and child development (CPS)
- [ ** ](85)
- Fetal alcohol spectrum disorder (FASD). CPS Position Statement
- [ ** ](86)
Adoption/Foster care: Children newly adopted or entering foster care are a high risk population with special needs for health supervision. Foster Care (CPS) | International Adoption: Preparing to adopt | International Adoption: Enhancing attachment
Immigrants/refugees: Caring for kids new to Canada (CPS) | CCIRH-Clinical Guidelines | Cross-cultural communication (CPS)
Aboriginal children: Social determinants of health in Aboriginal children in Canada (PCH)
- (DAN: same content 93 + 94) [ ** ](94)
Prevention of child maltreatment:
- Risk factors for child maltreatment:
- Parent (low socio-economic status, maternal age <19 years, single parent family, non-biological parents, abused as child, substance abuse, lack of social support, unplanned pregnancy or negative parental attitude towards pregnancy).
- Family (spousal violence, poor marital relations, poor child-parent relationship, unhappy family life).
- Child (behaviour problems, disability).
Discuss with parents of preschoolers teaching names of genitalia, appropriate and inappropriate touch, and normal sexual behaviour for age.
Exposure to personal violence and other forms of violence has significant impact on physical and emotional well-being of children.
Assess home visit need: There is good evidence for home visiting by nurses during the perinatal period through infancy for first-time mothers of low socioeconomic status, single parents or teenaged parents to prevent physical abuse and/or neglect.
Child maltreatment interventions (USPSTF) | Bruising in suspected maltreatment cases (CPS) | Abusive head trauma (CPS) | INSPIRE: 7 strategies for ending violence against children (WHO) - Risk factors for child maltreatment:
NONPARENTAL CHILD CARE
Inquire about current child care arrangements. High quality child care is associated with improved paediatric outcomes in all children.
Factors enhancing quality child care include: practitioner general education and specific training; group size and child/staff ratio; licensing and registration/accreditation; infection control and injury prevention; and emergency procedures.
- Health implications of children in child care centres (CPS): Part A and Part B
- Guide to child-care in Canada (CPS): Well Beings
LITERACY
Encourage parents to read and sing to their infants and children and to limit TV, video and computer games to provide more opportunities for reading.
FAMILY HEALTHY ACTIVE LIVING/SEDENTARY BEHAVIOUR/SCREEN TIME
Healthy active living (CPS) | CSEP guidelines | Screen time and young children (CPS)
Encourage increased physical activity, with parents as role models, through interactive floor-based play for infants and a variety of activities for young children, and decreased sedentary pastimes.
- Media use – Counsel on appropriate media use; for children <2 years, screen time (eg, TV, computer, electronic games) is not recommended except for video-chatting; for children 2-4 years, screen time should be limited to <1 h/day; less is better; educational and prosocial programming is better.
DEVELOPMENT
Manoeuvres are based on evidence-based literature on milestone acquisition. Evidence-based milestone ages (PCH). They are not a developmental screen, but rather an aid to developmental surveillance. They are set after the time of typical milestone acquisition. Thus, absence of any one or more items is considered a high-risk marker and indicates consideration for further developmental assessment, as does parental or caregiver concern about development at any stage. Assessment tools Table 4 (CPS) | Global Delay (CPS)
- Best Start website contains resources for maternal, newborn, and early child development
- Getting it right at 18 months (CPS) | Measuring in support of early childhood development (CPS)
- Identifying and treating speech & language delays (PCH) | Encyclopedia on Early Childhood Development
TOILET LEARNING
The process of toilet learning has changed significantly over the years and within different cultures. In Western culture, a child-centred approach is recommended, where the timing and methodology of toilet learning is individualized as much as possible.
Toilet learning (CPS)
Toilet-training strategy (PCH): Part A Part B
AUTISM SPECTRUM DISORDER
Specific screening for ASD at 18–24 months should be performed on all children with any of the following risk factors: failed items on the social/emotional/communication skills inquiry, sibling with autism, or developmental concern by parent, caregiver, or physician. Increased prevalence for ASD is also associated with prematurity, and certain chromosomal, genetic and neurological disorders. Standardized, evidence-based screening tools for detection of early ASD symptoms should be used as per guidelines. ASD (CPS): Early detection | Diagnostic assessment | Management | M-CHAT™
PHYSICAL EXAMINATION
- [ ** ](90)
Jaundice: Bilirubin testing (total and conjugated) if persists beyond 2 wks of age. Neonatal Hyperbilirubinemia Guidelines (CPS) | Newborn screening for biliary atresia (AAP).
- [ ** ](88)
Bruising: Unexplained bruising warrants evaluation re child maltreatment or medical illness.
- [ ** ](98)
Blood pressure: Check BP at all visits for those at risk > 3 yrs old. Some risk factors: obesity, sleep-disordered breathing, prematurity, renal disease, congenital heart disease, diabetes, or on med’ns that ^ BP.
High blood pressure in children, including definitions: (NIH Working Group) (AAP) - [ ** ](51)
Fontanelles: The posterior fontanelle is usually closed by 2 months and the anterior by 18 months. The Abnormal fontanel (AAFP)
- [ ** ](52)
Vision inquiry/screening: Vision screening (CPS)
- Check Red Reflex for serious ocular diseases such as retinoblastoma and cataracts.
- Corneal light reflex/cover–uncover test & inquiry for strabismus: With the child focusing on a light source, the light reflex on the cornea should be symmetrical. Each eye is then covered in turn, for 2–3 seconds, and then quickly uncovered. The test is abnormal if the uncovered eye “wanders” OR if the covered eye moves when uncovered.
- Check visual acuity at age 3–5 years.
- [ ** ](53)
Hearing inquiry/screening: Language delay or parental concerns about hearing acuity should prompt a rapid referral for hearing assessment. Formal audiology testing should be performed in all high-risk infants, including those with normal UNHS. Older children should be screened if clinically indicated.
- [ ** ](54) Inspect tongue mobility for ankyloglossia. CPS Position Statement
- (100)
Check palate for cleft Cleft lip/palate (AAP)
- [ ** ](55)
Tonsil size/sleep-disordered breathing: Screen for sleep problems. Behavioural sleep problems and snoring in the presence of sleep-disordered breathing warrants assessment re obstructive sleep apnea (OSA). OSA (AAP)
- (105)
Dental: Examine for problems including caries, oral soft tissue infections or pathology; and for normal teeth eruption sequence. Canadian Caries Risk Assessment Tool
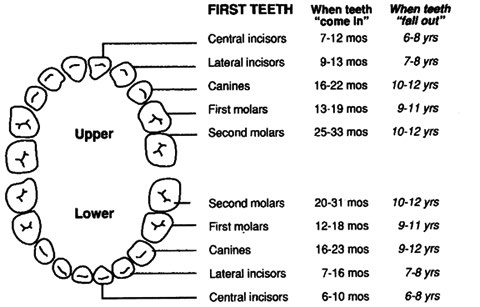
- [ ** ](91) Check neck for torticollis.
- (104)
Umbilicus: Gently pat dry and review S&S of infection. Preventing sepsis (Cochrane)
- [ ** ](57)
Hips: There is insufficient evidence to recommend routine diagnostic imaging for screening for developmental dysplasia of the hips, but examination of the hips should be included until at least one year, or until the child can walk. Exam includes assessing limb length discrepancy and asymmetric thigh or buttock (gluteal) creases; performing Ortolani manoeuvre (usually negative after 3 mos); and testing for limited abduction (usually positive after 3 mos). Consider selective imaging between 6 wks and 6 mos if risk factor (i.e. breech, family history, hip instability on physical exam). DDH (AAP)
- [ ** ](56)
Muscle tone: Assessment should be performed for abnormal tone or deep tendon reflexes, or for asymmetric movements (moving one side more than other). These may be early signs of cerebral palsy or neuromotor disorder and suggest the need for further assessment. CP Features (DM&CN)
- Spine/Anus: Examine spine for cutaneous signs of occult spinal dysraphism. Check anal patency. Congenital Brain and Spinal Cord Malformations (AAP)
INVESTIGATIONS/SCREENING
Anemia screening: Screening for iron deficiency anemia should be considered between 6 and 18 months of age for infants/children from high risk groups: E.g. Low SES; Indigenous communities; newly arrived refugee, internationally adopted and immigrant children from resource-poor countries; low-birth-weight and premature infants; infants/children fed whole cow’s milk before 9 months of age or at quantities > 500 mls/day; prolonged bottle feeding beyond 15 months of age; or sub-optimal intake of iron-containing foods. Beyond this age, anemia screening as per additional risk factors. Iron requirements (CPS)
Hemoglobinopathy screening: Screen all neonates from high-risk groups: Asian, African & Mediterranean.
Universal newborn hearing screening (UNHS): Effectively identifies infants with congenital hearing loss and allows for early intervention & improved outcomes. Universal newborn hearing screening (CPS)
Tuberculosis – TB skin testing: For up-to-date information, see Canadian TB Standards: 2014
ROUTINE IMMUNIZATION
See the Canadian Immunization Guide for recommended immunization schedules for infants, children, youth, and pregnant women, from the National Advisory Committee on Immunization (NACI)
Provincial/territorial immunization schedules may differ based on funding differences. Provincial/territorial immunization schedules are available at the Public Health Agency of Canada.
Immunization pain reduction strategies: During vaccination, pain reduction strategies with good evidence include breastfeeding or use of sweet-tasting solutions, giving the most painful vaccine last, and consideration of topical anaesthetics.
Reducing vaccine pain (CMAJ)Acetaminophen or ibuprofen should not be given prior to, but after vaccination as required. Prophylactic Antipyretic Administration (PLOS ONE)
Information for physicians on vaccine safety:
Information for parents on vaccinations can be accessed through:
VACCINE NOTES
(Adapted from websites of NACI and the Canadian Immunization Guide September 2019)
Diphtheria, Tetanus, acellular Pertussis, inactivated Polio virus vaccine, and Haemophilus influenzae B (DTaP-IPV-Hib): DTaP-IPV-Hib vaccine may be used for all doses in the vaccination series in children < 2 years of age, and for completion of the series in children < 5 years old who have received ≥ 1 dose of DPT (whole cell) vaccine (e.g. recent immigrants).
Diphtheria, Tetanus, acellular Pertussis, inactivated Polio virus vaccine, Haemophilus influenzae B, and Hepatitis B (Hep B) (DTaP-IPV-Hib-Hep B) is used for 3 of the 4 initial doses in some jurisdictions with routine infant Hep B vaccination programs.
Diphtheria, Tetanus, acellular Pertussis, inactivated Polio virus vaccine (DTaP-IPV) may be used up to age 7 years and for completion of the series in incompletely immunized children 5-7 years old (healthy children ≥5 years of age do not require Hib vaccine).
Haemophilus influenzae type b conjugate vaccine (Hib): Hib is usually given as a combined vaccine (DTaP-IPV-Hib above). If required and not given in combination, Hib is available as Haemophilus b capsular polysaccharide – PRP conjugated to tetanus toxoid (Act-HIBTM or HiberixTM). The number of doses required depends on the age at vaccination and underlying health status.
Diphtheria, Tetanus, acellular Pertussis, inactivated Polio virus vaccine (DTaP-IPV) may be used up to age 7 years and for completion of the series in incompletely immunized children 5-7 years old (healthy children ≥5 years of age do not require Hib vaccine).
Tetanus, Diphtheria, Pertussis, Polio (Tdap-IPV) Vaccine, a quadrivalent vaccine containing less pertussis and diphtheria antigen than the preparations given to younger children and less likely to cause local reactions, is used for the preschool booster at 4-6 years of age in some jurisdictions and should be used in all individuals > 7 years of age receiving or completing their primary series.
Diphtheria, Tetanus, acellular Pertussis vaccine (dTap) is used for booster doses in people ≥ 7 years of age. All adults should receive at least one dose of pertussis containing vaccine (excluding the adolescent booster). Immunization with dTap should be offered to all pregnant women (≥13 weeks of gestation, ideally at 27 – 32 weeks) to provide immediate protection to infants less than 6 months of age.
Rotavirus vaccine: Universal rotavirus vaccine is recommended by NACI and CPS. Two oral vaccines are currently authorized for use in Canada: Rotarix (2 doses) and RotaTeq (3 doses). Dose #1 is given between 6 weeks and 14 weeks+6 days with a minimum interval of 4 weeks between doses. Maximum age for the last dose is 8 months/0 days.
Recommendations for the use of rotavirus vaccines in infants (CPS)
Measles, Mumps and Rubella vaccine (MMR), and MMR-varicella (MMRV): The first dose is given at 12-15 months and a second dose should be given with the 18 month or preschool dose of DTaP-IPV (±Hib) (depending on the provincial/territorial policy), or at any intervening age that is practical but at least 4 weeks after the first if MMR, or 3 months after the first if MMRV. If MMRV is not used, MMR and varicella vaccines should be administered concurrently, at different sites, or separated by at least 4 weeks.
Varicella vaccine: Children aged 12 months to 12 years who have not had varicella should receive 2 doses of varicella vaccine (univalent varicella or MMRV). Unvaccinated individuals ≥ 13 years who have not had varicella should receive two doses at least 28 days apart (univalent varicella only). Consult NACI guidelines for recommended options for catch-up varicella vaccination. Varicella and MMR vaccines should be administered concurrently, at different sites if the MMRV [combined MMR/varicella] vaccine is not available, or separated by at least 4 weeks. Preventing varicella (PCH)
Hepatitis B vaccine (Hep B):
- Hepatitis B vaccine can be routinely given to infants or preadolescents, depending on the provincial/territorial policy. The first dose can be given at 1 month, or at 2 months of age to fit more conveniently with other routine infant immunization visits. The second dose should be administered at least 1 month after the first dose, and the third at least 2 months after the second dose, but again may fit more conveniently into the 4- and 6-month immunization visits. Alternatively, Hep B can be administered as DTaP-IPV-Hib- HepB vaccine in infants, with the first dose at 2 months of age. A two-dose schedule for adolescents is an option.
- For infants born to a mother with acute or chronic hepatitis B (HBsAg-positive), the first dose of Hep B vaccine should be given at birth (with Hepatitis B immune globulin) and repeat doses of vaccine at 1 and 6 months of age. Premature infants of birthweight less than 2,000 grams, born to HB- infected mothers, require four doses of HB vaccine at 0, 1, 2, and 6 months. The last dose should not be given before 6 months of age. Infants of HBsAg- positive mothers also require Hepatitis B immune globulin at birth and follow-up immune status at 9–12 months for HBV antibodies and HBsAg.
- Infants with HBsAg-positive fathers, siblings, or other household contacts require Hepatitis B vaccine at birth, and at 1 month, and 6 months of age.
- Hepatitis B vaccine should also be given to all infants from high-risk groups, such as:
- infants where at least one parent has emigrated from a country where Hepatitis B is endemic;
- infants of mothers positive for Hepatitis C virus;
- infants of substance-abusing mothers.
- For children in medically high risk groups (e.g. immunocompromising conditions, chronic renal failure, dialysis), see Hepatitis B chapter in the Canadian Immunization Guide for schedules re: timing and number of Hep B vaccine doses and monitoring of HB antibody levels.
Hepatitis A or A/B combined (HAHB - when Hepatitis B vaccine has not been previously given):
- Children 6 months and older in high-risk groups should receive 2 doses of the hepatitis A vaccine given 6-36 months apart (depending on product used). HAHB is the preferred vaccine for individuals with indications for immunization against both hepatitis A and hepatitis B, who are ≥12 months unless medical condition indicates high dose Hep B vaccine required.
- These vaccines should also be considered when traveling to countries where Hepatitis A or B are endemic.
- Possible HAHB schedules include 12 months to 18 years: 2 doses at months 0 and 6-12; OR 3 doses at months 0, 1, and 6 depending on age and product used.
Pneumococcal vaccine: conjugate (Pneu-C-13) and polysaccharide (Pneu-P-23): Recommended schedule, number of doses and product depend on the age of the child, risk for pneumococcal disease, and when vaccination is begun. Consult NACI guidelines. Routine infant immunization: administer three doses of Pneu-C-13 vaccine at minimum 8-week intervals beginning at 2 months of age, followed by a fourth dose at 12 to 15 months of age. For healthy infants, a three-dose schedule may be used, with doses at 2 months, 4 months, and 12 months of age. Children 2 years and above who are at highest risk of invasive pneumococcal disease should receive Pneu-P-23. Consult NACI guidelines for eligibility and dosing schedule.
Meningococcal vaccine:
- Canadian children should be immunized with a MCV-C at 12 months of age, or earlier depending on provincial/territorial vaccine programs; suggested one dose at 12 months of age.
- MCV-4 (A, C, Y, W) should be given to children two months of age and older who are at increased risk for meningococcal disease or who have been in close contact with a case of invasive meningococcal A,C,Y or W disease. MCV-4-CRM (MenveoTM) should be used for those less than 2 years old; any MCV-4 may be used for older children.
- A routine booster dose with MCV-4 or MCV-C is recommended at approximately 12 years of age. High risk children require boosters at 5 year intervals.
- MCV-4 should be given to children two months of age and older travelling to areas where meningococcal vaccine is recommended. MCV-4 CRM is recommended for immunization of children 2 months to less than 2 years of age. Any MCV-4 may be used for older children.
- Multi-component meningococcal serogroup B (4CMenB) vaccine should be considered for active immunization of children ≥ 2 months of age who are at high risk of meningococcal disease or who have been in close contact with a case of invasive meningococcal B disease or travelling to an area where risk of transmission of meningococcus B is high. Two to 3 doses are required at 4 or 8 wk intervals depending on age.
- Routine prophylactic administration of acetaminophen after immunization and/or separating 4CMenB vaccination from routine vaccination schedule may be considered for preventing fever in infants and children up to 3 years of age.
Influenza vaccine: Recommended for all children, particularly those aged 6-59 months and other children at high risk.
- Previously unvaccinated children up to 9 years of age require 2 doses with an interval of at least 4 weeks. The second dose is not required if the child has received one or more doses of influenza vaccine during the previous immunization season. A quadrivalent vaccine should be used if available.
- For children between 6 and 23 months, the quadrivalent inactivated influenza vaccine (QIV) should be used, and if not available, either unadjuvanted or adjuvanted trivalent inactivated vaccine (TIV).
- Children 2-18 years of age should be given QIV, or quadrivalent live attenuated influenza vaccine (LAIV) if not contraindicated. If a quadrivalent vaccine is not available, TIV should be used. Egg allergy is not a contraindication to vaccination with QIV, TIV, or LAIV.
- Immunization with TIV or QIV in the second or third trimester to provide protection for the pregnant woman and infant < 6 months of age.
- LAIV is contraindicated for children i) with immune compromising conditions, ii) with severe asthma (defined as current active wheezing or currently on oral or high-dose inhaled glucocorticosteroids, or medically attended wheezing within the previous 7 days), or iii) on aspirin.
Respiratory syncytial virus (RSV) vaccine: Palivizumab (Synagis) prophylaxis during RSV season for children with chronic lung disease, congenital heart disease or born preterm. Preventing hospitalizations for respiratory syncytial virus infection (CPS)
